Abstract
Background: Race/ethnicity are associated with varied outcomes in MM (Ailawadhi S, et al. Br J Haematol. 2012;158:91-98). Because many previous MM clinical trials had predominantly white (W) participants, the reported clinical effects of therapeutic interventions for MM may not be generalizable to patients of other races/ethnicities. Prior data has suggested that racial/ethnic minorities may not have adequate access to novel anti-MM therapies (Kirtane K, Lee S. Blood . 2017 Jul 19 [Epub]). Few prospective studies, and none in the setting of novel agents, eg, IMiD compounds, have explored the effect of race/ethnicity on MM outcomes. This subgroup analysis of the MM-009 phase 3 clinical trial compared the progression-free survival (PFS) benefit experienced by African American (AA) vs W patients treated with lenalidomide (LEN). Safety was also evaluated.
Methods: In the MM-009 trial, 353 patients from the United States and Canada who had received ≥ 1 previous therapy for MM but still experienced disease progression were randomized to treatment with either LEN plus dexamethasone (LEN-D; n = 177) or placebo plus dexamethasone (PBO-D; n = 176). LEN 25 mg or PBO was given on days 1-21 of a 28-day cycle. Both treatment groups received D 40 mg on days 1-4, 9-12, and 17-20 of the first 4 cycles, then days 1-4 for cycle 5 and beyond. The median follow-up time for surviving patients was 26.2 months in the LEN-D group and 12.9 months in the PBO-D group. In this analysis, the baseline characteristics and outcomes of 42 AA and 289 W patients were compared. Data from 22 patients (11 from each treatment arm) were excluded because race was reported as other than W or AA.
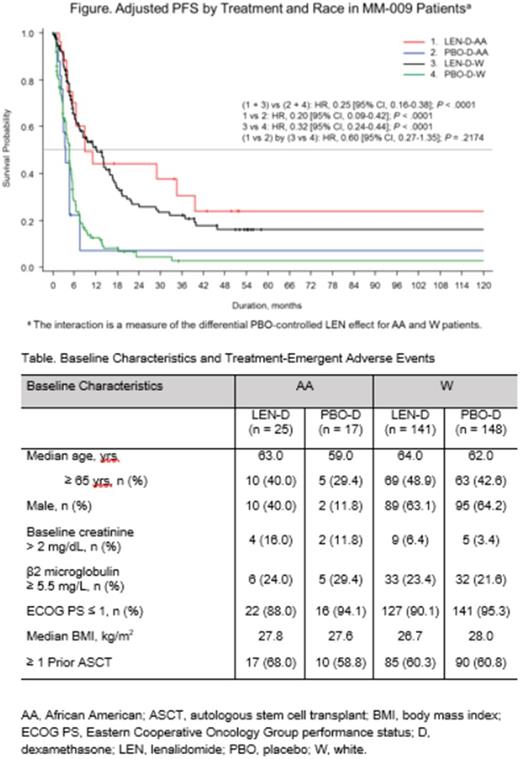
Results: Patients were analyzed in 4 groups, by treatment and by race: LEN-D-AA (n = 25), PBO-D-AA (n = 17), LEN-D-W (n = 141), and PBO-D-W (n = 148). Baseline characteristics were similar across the AA and W groups, except for sex (P < .0001) and baseline creatinine (P= .0172). Male patients represented 63.7% and 28.6% of the W and AA patient populations, respectively. A greater proportion of patients in the AA (14.3%) than the W group (4.8%) had baseline creatinine > 2 mg/dL. Detailed baseline characteristics are listed in the table. LEN treatment decreased risk of disease progression or death in both the AA (HR, 0.20 [95% CI, 0.09-0.42]; P < .0001) and the W (HR, 0.32 [95% CI, 0.24-0.44]; P <.0001) groups compared with PBO (Figure). Median PFS was 9.2, 3.7, 13.1, and 4.6 months in the LEN-D-AA, PBO-D-AA, LEN-D-W, and PBO-D-W groups, respectively. The most common hematologic treatment-emergent adverse event (TEAE) was neutropenia, which occurred in 52.0% of patients in the LEN-D-AA group, 11.8% of patients in the PBO-D-AA group, 46.1% of patients in the LEN-D-W group, and 6.8% of patients in the PBO-D-W group. Fatigue was the most common nonhematologic TEAE, occurring in 52.0%, 23.5%, 51.8%, and 52.0% of patients in the LEN-D-AA, PBO-D-AA, LEN-D-W, and PBO-D-W groups, respectively.
Conclusions: The data presented here indicate that LEN administration for the treatment of RRMM results in a similar PFS benefit in AA patients as in W patients. It is imperative to provide adequate access of novel anti-MM agents to racial minorities.
Ailawadhi: Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pharmacyclics: Research Funding. Larkins: Celgene: Employment. Chung: Celgene Corporation: Employment, Equity Ownership. Srinivasan: Celgene: Employment. Agarwal: Celgene Corporation: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal